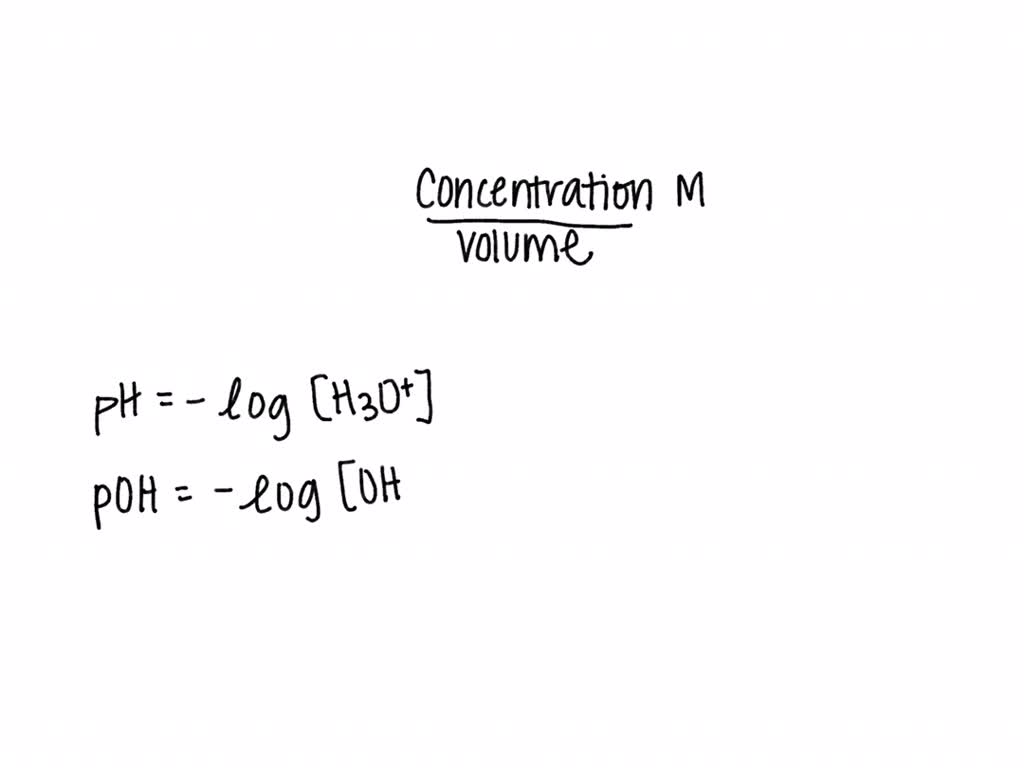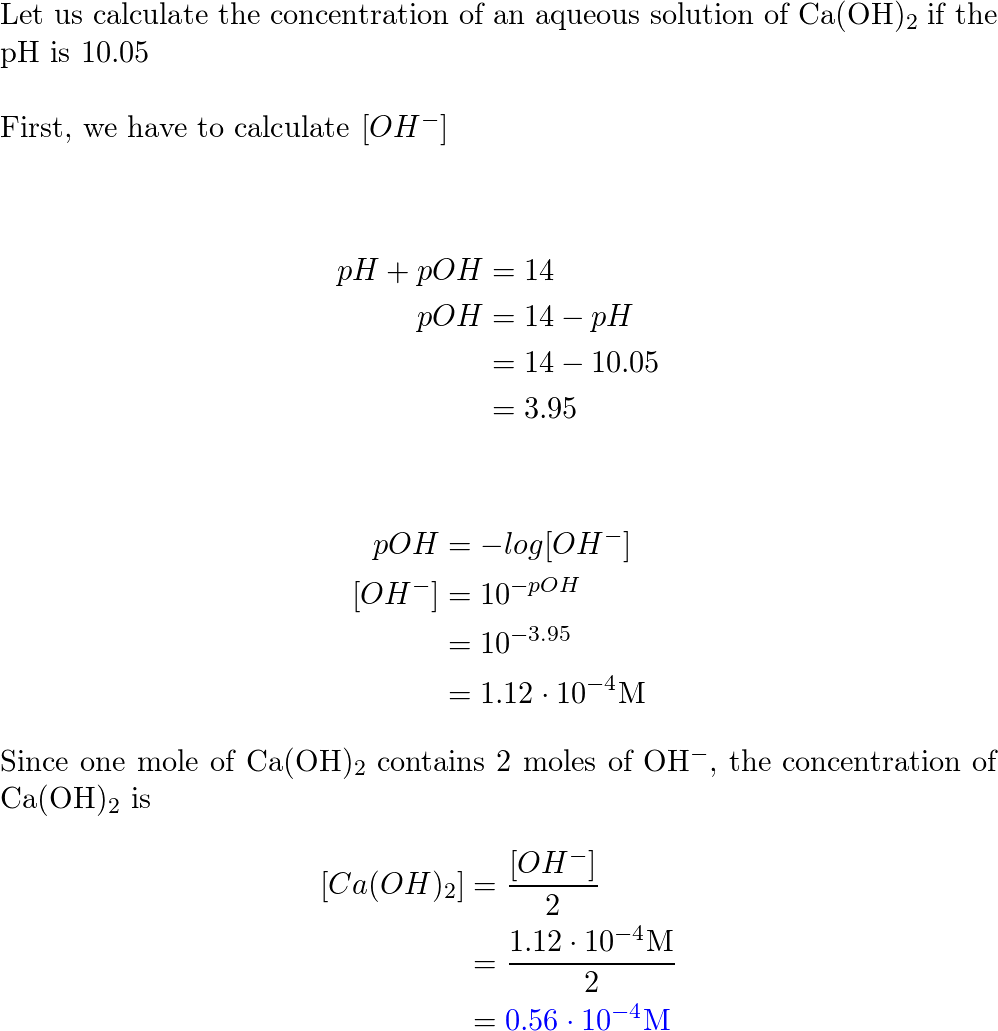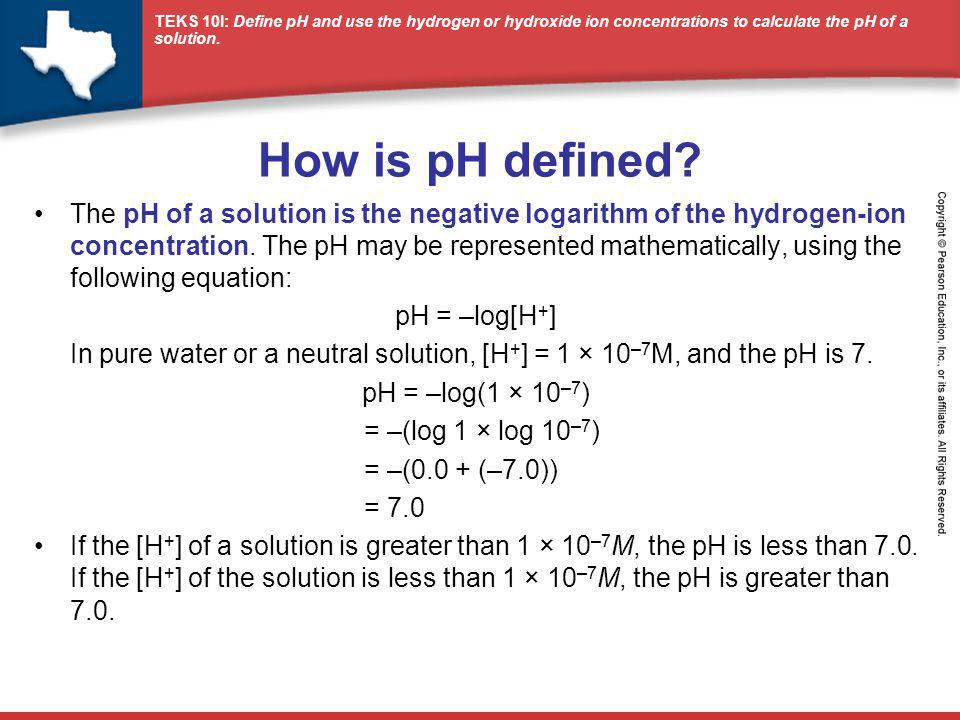
How is pH defined? The pH of a solution is the negative logarithm of the hydrogen-ion concentration. The pH may be represented mathematically, using the. - ppt video online download
What is the concentration of hydrogen ion and hydroxide ions of solution which has pH of 4.87? - Quora
✓ Solved: Calculate the concentration of an aqueous HBr solution that has pH = 4.25. HBr is a strong...
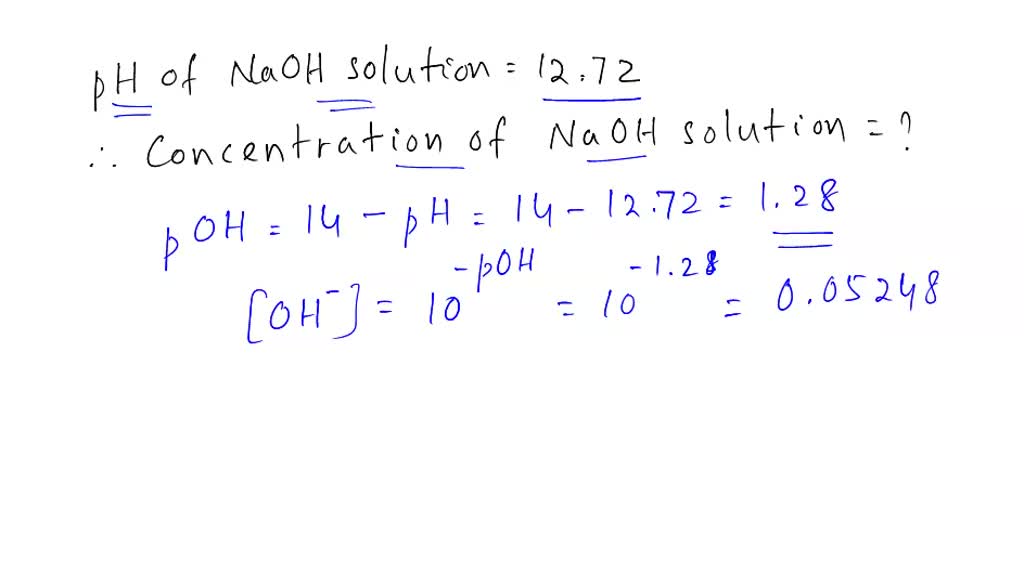




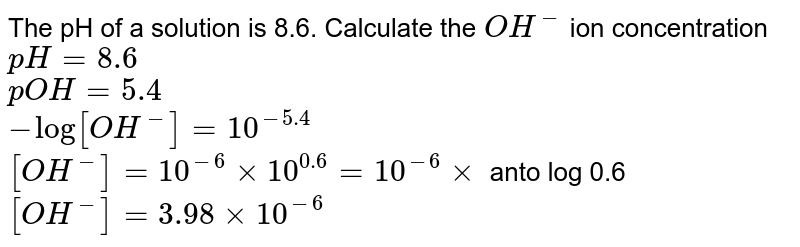








![Calculations of pH, pOH, [H+] and [OH-] Calculations of pH, pOH, [H+] and [OH-]](https://www.sciencegeek.net/Chemistry/taters/graphics/pHSchematic.gif)